Research
We study organization and mechanics of subcellular structures including the nucleus and the actin cytoskeleton to address a wide range of medically-relevant questions. Focusing on our strengths incorporating physics and engineering into complex biological questions has led us to high-impact results and a large range of biomedical applications.
Fundamental studies linking nuclear rheology and gene expression:
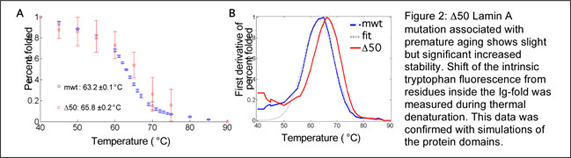
Thermodynamics of nuclear structural proteins and disease-causing mutants:
From our studies on nuclear rheology, we have become particularly interested in a premature aging syndrome. Several years ago we found that the mutant ?50 lamin A accumulated at the nuclear membrane and stiffened the nucleus (Dahl, et al. PNAS 2006). To better determine the mechanism of membrane accumulation, we express recombinant proteins (in E.coli, insect cells and human cells), purify and characterize the thermodynamic stability of these proteins (Figure 2).
With the purified protein, we are also quantifying binding to membranes. Generally, these studies provide (a) molecular insight into protein lipidation, (b) structure and characterization of intrinsically disordered proteins, (c) theoretical insight and experimental quantification of protein-protein, -DNA, and -membrane binding. We have also discovered potential therapeutic targets related to premature aging that we are testing in patient cells.
We are also studying the etiology of Emery Dreyfus muscular dystrophy (EDMD) by examining unique mechanical features associated with accessory nuclear structural proteins. This work has also generated significant interest since we have discovered a potential mechanism for EDMD progression based on a previously undiscovered mechanical phenotype. This is another example of how our cross-disciplinary approach has generated high impact findings.
Characterization of stem cell mechanics for design and development of delivery systems:
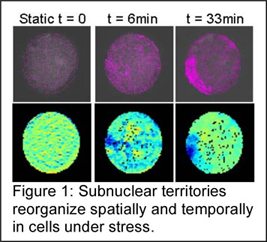
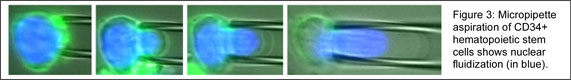
Expanding our functional understanding of nuclear structure and mechanics we have begun to characterize mechanics of adult stem cells and the contributions of subcellular structures. Currently we are investigating deviations in mechanics and differentiation potential of bone-marrow derived mesenchymal stem cells to develop better clinical injection procedures. This collaboration with clinicians at the Cleveland Clinic, we are engineering design systems and protocols to optimize cell survival. We are also investigating the utility of using hematopoietic stem cells for injection since they have unique rheology and subcellular structures fluidize under high shear (Figure 3).
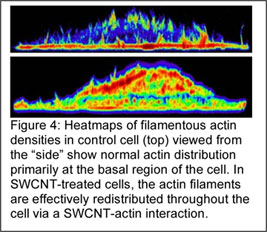
Interactions of actin structures and carbon nanotubes for in situ imaging and actuation: